Abstract
Background: Therapy-related myeloid neoplasms (tMN) is a second haematological malignancy associated with distinct molecular profile (Singhal et al Leukemia 2019) and dismal outcome. tMN is believed to arise from cytotoxic DNA damage to haematopoietic stem cells (HSC). Although, cytotoxic therapy (CT) can damage bone marrow (BM) microenvironment, its role in tMN pathogenesis remains unknown.
Aim and Methods: We performed comprehensive multiomic profiling (transcriptomic, cytokine quantification, phenotype, DNA damage) of BM stromal cells (BMSC) from (i) tMN patients previously exposed to CT. Critically, we compared it with (ii) patients with MN and a history of another cancer without CT (pMN+Ca), (iii) primary MN (pMN) and (iv) age-matched controls (Healthy).
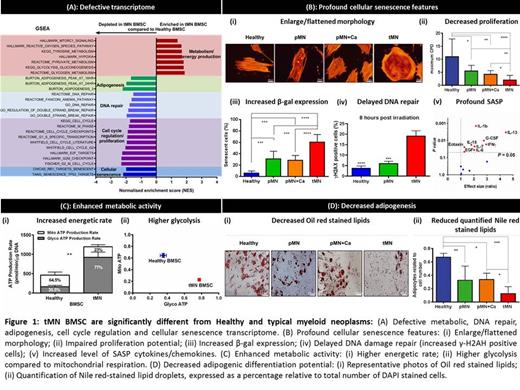
Results: To decipher microenvironmental changes induced by CT from that of MN and age-related changes, whole transcriptome analysis was performed on BMSC isolated from tMN and compared with all control cohorts. Twenty-nine genes were differentially expressed in tMN compared to Healthy (FDR < 0.1, P < 0.05). Unexpectedly 146 genes were differentially expressed in tMN BMSC compared to other MN and interestingly, ~90% of differentially expressed genes were involved in senescence. Moreover, functional enrichment and GO analysis suggest DNA damage repair, cell cycle regulation, and senescence pathways were deregulated in tMN BMSC (Fig. 1A). Genes such as CDKN1A (a critical cyclin dependent kinase inhibitor orchestrating cell cycle arrest), TNFRSF10D (senescence associated), and FGF-2 (a key player in cell proliferation) were highly expressed (P < 0.001).
These findings were validated by demonstrating other features of senescence in tMN BMSC: (i) enlarged/flattened cellular morphology, (ii) decreased cell proliferation and colony-forming potential, (iii) increased β-galactosidase expression, and (iv) defective DNA damage repair (Fig. 1Bi-iv). Interestingly, within the tMN cohort there was no correlation between latency period (the interval between completion of CT until tMN diagnosis) and senescence, indicating that higher senescence is persistent even after several years of CT.
Senescence associated secretory phenotype (SASP), a mixture of inflammatory cytokines and chemokines such as IL-7, IL-1β, IL-13, and IL-6, were significantly higher in conditioned media of tMN BMSC (9/14, 64%) (Fig. 1 Bv).
Despite reduced proliferation and senescence, transcriptome analysis showed enrichment of metabolic and energy production pathways in tMN BMSC compared to controls. TXNRD1, regulator of glucose and lipid metabolism, and BNIP3, a negative regulator of mitochondrial potential, were highly expressed in tMN BMSC (P < 0.001). These findings were further verified by Seahorse bioenergetic analyses. The overall energetic rate (as assessed by ATP production) was higher in tMN compared to Healthy BMSC (P = 0.002), with higher proportion of ATP generated by glycolysis (77% versus 35.5%) (Fig. 1C).
Adipogenic differentiation potential of senescent BMSC is not well known. Transcriptome analysis showed reduced expression of genes involved in adipogenesis in tMN BMSC. This was further validated by two independent in vitro assays showing reduced adipogenesis (Fig. 1D). Interestingly, PNPLA2, a catalyst of the first lipolysis reaction, were significantly de-regulated in tMN BMSC (P < 0.001).
The key difference in tMN and other MN is prior exposure to CT. Hence, we hypothesise that prior CT leads to long-term irreversible damage to BM microenvironment and induced senescence, which in turn propagate senescence in surrounding normal cells and promote clonal abnormalities in HSC. Other possibility is that tMN clone can induce these changes in BM microenvironment. To decipher it, we assessed serial BMSC. We observed aberrant stroma proliferation and bi-differentiation capacity, following CT, well before the diagnosis of tMN.
Conclusions: By multiple orthogonal indices, our results show that tMN BMSC lie on an extreme trajectory away from normal and typical MN, with massive defect in senescence and distinct metabolic phenotype. Importantly, prior CT leads to long-term irreversible damage to the BM microenvironment which potentially contributes to tMN pathogenesis. Together, these data provide a valuable resource for future strategies to delay or prevent the onset of tMN and assist in marrow regeneration in patients undergoing CT.
Hughes: BMS: Research Funding; Novartis: Honoraria, Research Funding; Takeda: Honoraria. Hiwase: Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal